In recent Grainews issues, I have discussed developing nitrogen (N), phosphorus (P) and potassium (K) fertilizer recommendations. I will continue with this theme and discuss developing sulphur (S) fertilizer recommendations in this column.
Sulphur deficiency was first observed in Alberta more than 90 years ago on grey wooded soils. Over the past 30 years, sulphur deficiency has become increasingly common and widespread across the Prairies as farmers have shifted to direct seeding, continuous cropping, using more diverse crop rotations and growing crops with higher sulphur requirements such as canola. It is estimated 35 million acres are considered potentially sulphur deficient across Alberta, Saskatchewan and Manitoba.
Read Also

PMRA denies strychnine emergency use request
Emergency use of strychnine for the 2026 growing season has been denied by the Pest Management Regulatory Agency.
Sulphur is involved in various roles in plants. It is important in protein formation in cereals. Sulphur is required in the development of fertile canola flowers and is needed for nodule development of legume and pulse crops.
Plants take up sulphur from soil in the form of sulphate-sulphur (SO4-S). Sulphur deficiency can be easily corrected with sulphate-sulphur-containing fertilizers or elemental sulphur fertilizers with very small particle size for conversion to sulphate-sulphur. For many Prairie farmers, sulphur is the third-most-limiting soil nutrient after nitrogen and phosphorus for cereal and oilseed crops in Western Canada.
Most of the sulphate-sulphur in surface soil is derived from soil organic matter breakdown. It is important to note sulphate-sulphur has a negative charge and will not attach to soil particles. Therefore, sulphate is fairly mobile in soil. It can leach downward in soil with excess precipitation, particularly in coarse-textured soils. Oilseed and forage crops tend to have a higher sulphur requirement than cereal or pulse crops.
Thin black, black and grey wooded soils tend to be more prone to sulphur deficiency. These soils are in areas of higher rainfall with greater leaching potential. These soils have higher yield potential due to higher precipitation and lower evapotranspiration levels. Higher crop yields tend to result in greater sulphur uptake and removal by crops.
Soils that are sandy or low in organic matter tend to be more prone to sulphur deficiency, as lower amounts of sulphate-sulphur are released from organic matter. Upper and mid-slope field positions tend to be lower in sulphate-sulphur in fields with variable topography.
Brown and dark brown soils of the southern Prairies often have an abundance of sulphate-sulphur in subsoil due to the natural presence of gypsum salts, or calcium sulphate (CaSO4). Gypsum can be an important source of plant-available sulphur in these soils.
In the past, the brown and dark brown soil zones were not considered sulphur deficient because of relatively high levels of gypsum in subsoil. Research has clearly shown that deficient levels of sulphate-sulphur occasionally occur in surface soil, and crops will respond to the addition of sulphur fertilizer, even though the subsoil has adequate plant-available sulphate-sulphur levels.
Irrigation farmers generally do not have to worry about sulphur deficiency. Water used for irrigation that originates from the Rocky Mountains naturally contains sulphate-sulphur. As a general rule, about 30 pounds per acre of plant-available sulphate-sulphur is applied to soil in 12 inches of irrigation water. Usually, the sulphur applied in irrigation water is sufficient to meet crop requirements over a growing season. Irrigation farmers should soil test irrigated fields to ensure sulphate levels are adequate in surface soil each spring before seeding.
Across the Prairies, the combinations of coarser-textured soils, high rainfall, high crop sulphur removal, lower organic matter and/or topographic position of soils on the landscape can predispose soils to sulphur deficiency. Farmers need to be aware of potential soil conditions that tend toward sulphur deficiency on their land.
Soil testing for sulphate-sulphur
Obtaining representative soil samples to assess sulphate-sulphur levels from a variable field can be a challenge. Research studies across all Prairie soil zones have found the level of plant-available sulphur can vary greatly with landscape across a field. Using benchmark, topographic or soil management zone soil sampling can be helpful to determine soil sulphur levels and to develop fertilizer plans. Carefully sampling each soil management zone in a field is a wise approach. If sampling locations are not representative, misleading information could lead to overapplication or underapplication of sulphur fertilizer.
Saline soil areas have elevated sulphate-sulphur levels. Never include soil samples from even a slightly saline area with normal field soil samples to avoid skewed results.
Plant-available sulphate-sulphur in soil is determined by measuring the soluble sulphate-sulphur content in soil samples from zero- to six-, six- to 12- and 12- to 24-inch depths or from zero- to six- and six- to 24-inch depths. As mentioned, sulphate is mobile and frequently occurs in larger amounts in subsoil. Therefore, incremental depth soil sampling is very important. Carefully taken soil samples can provide a fairly reliable basis for making sulphur fertilizer recommendations. But it is critically important that representative soil samples be obtained from the field.
Sulphur fertilizer recommendations
Table 1 (below) provides my suggested guide to the interpretation of soil test results. Consult your provincial agriculture department website for more detailed information on recommended sulphur for specific crops.
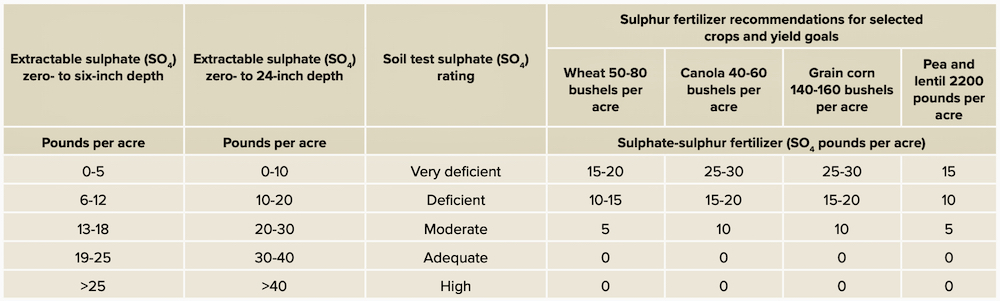
It is important to look at both the zero- to six-inch depth and the combined zero- to 24-inch depths for correct interpretation. Start by looking at the zero- to six-inch depth to decide if the surface soil is deficient. Then, look at the combined zero- to 24-inch-depth samples. If one or both are deficient, sulphur fertilizer is likely needed. Remember, it is relatively common to have adequate sulphate-sulphur within the combined zero- to 24-inch depth, but when the zero- to six-inch depth is low in plant-available sulphur, sulphate fertilizer is needed to ensure adequate crop establishment and root growth until the roots have penetrated the subsoil.
Sulphate-sulphur fertilizers have a relatively high salt index, so be very careful not to overapply seed-placed sulphate fertilizer. For cereal crops, the maximum safe rate of sulphate-sulphur that can be applied with the seed with a 10 per cent seedbed utilization (SBU) is about 15 to 20 pounds per acre of sulphate-sulphur, assuming not more that 30 pounds per acre of phosphate fertilizer is also seed-placed, depending on soil moisture conditions and the opener type used.
The safe seed-placed sulphate-sulphur rate for canola should not exceed 10 pounds per acre when using a 10 per cent SBU, assuming 10 pounds per acre of phosphate fertilizer is also seed-placed. For canola, it is often best to seed-place the safe rate of phosphate and side or mid-row band sulphate and other fertilizers.
Some single or double disc no-till drills cut a narrow furrow, placing the seed and fertilizer in very close proximity in the bottom of the seed furrow. This may create a concentrated fertilizer band with the seed with a SBU as low as five per cent, which could damage germinating seed.
Sulphur fertilizers
Sulphur fertilizers are either in sulphate-sulphur form or as elemental sulphur (S°) fertilizer products. Ammonium sulphate (21-0-0-24) fertilizer contains sulphate and is excellent for crops grown on sulphur-deficient soils. Ammonium sulphate dissolves quickly, provided there is adequate soil moisture to release sulphate-sulphur for uptake by plants. Other fertilizer products, such as potassium sulphate, can be used to correct both potassium and sulphur deficiencies. Unfortunately, most dealers do not handle this fertilizer product as it tends to be relatively expensive.
There are several elemental sulphur products available. Elemental products are relatively inexpensive and are becoming increasingly popular. It is important to note that elemental sulphur products are not in a plant-available form and elemental sulphur cannot be taken up by plants.
Elemental sulphur fertilizer must be oxidized by very specific soil microbes to convert to sulphate-sulphur. The rate of conversion from elemental sulphur to plant-available sulphate-sulphur depends on the particle size and the method of application. Recent research in Alberta showed that elemental sulphur with a mean particle size of 20 microns (one micron is one-millionth of a metre) converts relatively quickly to sulphate. The study showed liquid Sulgro 70 sprayed on the soil surface before seeding oxidized appreciably during the first eight weeks after seeding and with suitable management has the potential to meet crop sulphur requirements in the year of application.
Granular elemental sulphur products must be carefully managed to be effective. Ideally, an elemental sulphur fertilizer should have a mean particle size of less than 75 microns. Elemental products with a mean micron size of greater than 150 microns may take several years or more to convert to sulphate-sulphur. Before purchasing an elemental sulphur fertilizer, ask the dealer the mean sulphur particle size of the product. For best management, granular elemental sulphur products should be surface broadcast without incorporation the fall before benefit is needed. Elemental sulphur broadcast on the soil surface should disperse on the surface and be more easily oxidized by soil microbes because rainfall and freeze-thaw cycles break down and disperse the elemental sulphur granules.
















