A new report from the World Health Organization’s cancer research agency places a herbicide well known to Canadian corn growers in the same potential-hazard category where the agency put glyphosate.
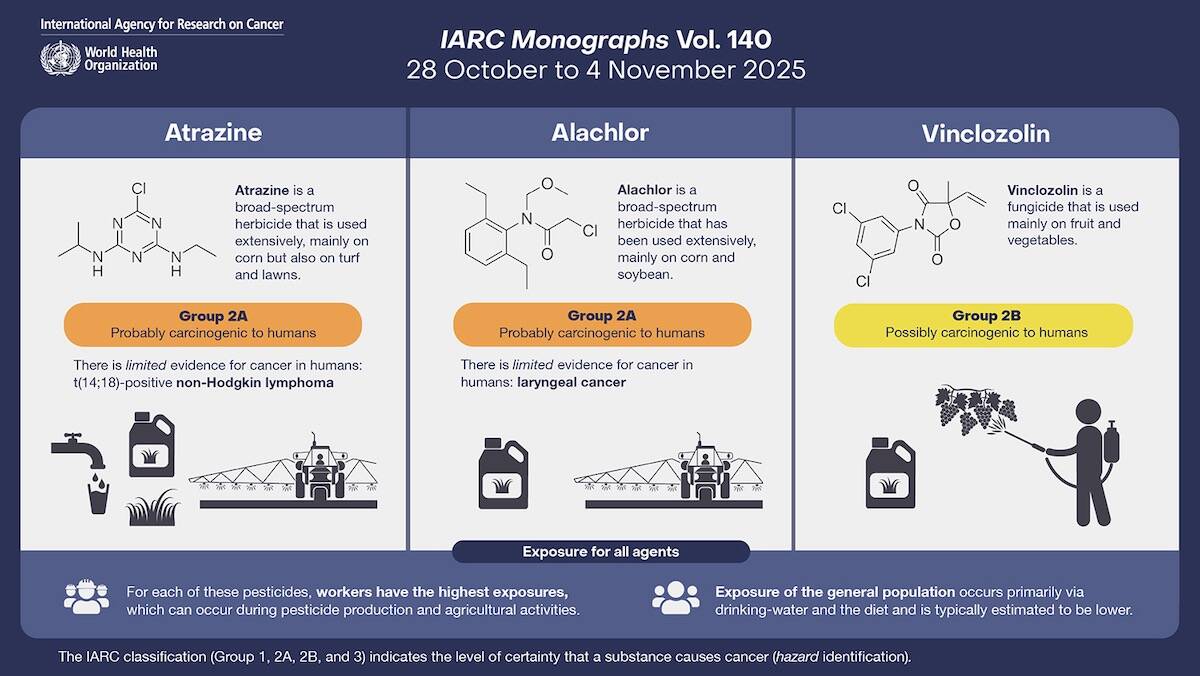
The International Agency for Research on Cancer (IARC) on Nov. 21 released a new monograph evaluating the carcinogenicity of atrazine, and reclassifying the chemical into IARC Group 2A, or “probably carcinogenic to humans.”
Atrazine, an off-patent triazine chemical from herbicide Group 5 (PSII inhibitors), has been on the market in Canada since 1960.
Read Also

India likely to triple lentil import duty
Analysts anticipate India hiking duties to 30 per cent after March 31 to bolster domestic prices on expectation of strong harvest.
Today it’s a registered active ingredient in herbicides including Syngenta’s AAtrex, Acuron and Primextra II Magnum; BASF’s Frontier Max and Marksman; and Bayer’s Converge 480, among others, mainly for use against broadleaf weeds in corn crops.
WHY IT MATTERS: On top of the points raised here about atrazine’s past and present hazard status, an unfavourable IARC ruling may potentially affect the herbicide’s future.
Known effects and measures
Research of atrazine has long shown that, at certain levels, the chemical can potentially cause harmful effects in people and other mammals.
In Syngenta’s Canadian safety data sheet (MSDS) for AAtrex Liquid 480, for example, it notes atrazine “has been reported to cause tumours in certain animal species,” but “there is no evidence that these findings are relevant to humans.”
It also notes AAtrex “may cause damage to organs (heart, kidney) through prolonged or repeated exposure.”
In Canada, the product has undergone multiple reviews — most recently a Health Canada special review to follow up on information received in a previous special review in 2017. That information related to the “potential changes to toxicology endpoint(s) used for previous human health and environmental risk assessments.”
In its latest proposed special review decision, released in early 2023, Health Canada said its review of data “re-confirmed that the most sensitive effect atrazine can cause is to suppress luteinizing hormone,” which, in tests with Sprague-Dawley laboratory rats, caused mammary gland tumours.
However, Health Canada said, the reproductive aging that causes mammary gland tumours to spontaneously form in some strains of rats is in humans instead defined by a lower number of egg cells in ovaries, not by suppression of luteinizing hormone.
Furthermore, Health Canada said, other than in the Sprague-Dawley strain of rats, such tumours weren’t observed in any other strains of rats or mice tested. Thus, Health Canada concluded there was “no evidence to link atrazine with breast cancer effects on human health.”
To help reduce the risks of atrazine exposure to other mammals, birds, fish, insects and non-target plants, Health Canada’s proposed decision also calls for new spray buffer zones of 15 metres in corn and switchgrass crops and 10 metres in sorghum crops.
Among other updates, to limit human exposure, Health Canada also proposes to:
- cancel the registered use of incorporating atrazine into granular fertilizer in commercial fertilizer plants;
- require a closed mixing/loading system when more than 85 kg of atrazine is used in a day;
- require a closed cab when applying more than 133 kg of atrazine in a day; and
- update the precautionary statements on the label, adding information on how to reduce sprays drifting to nearby areas where people live, and restrictions on people’s entry into areas treated with atrazine.
All that said, Health Canada noted it only approves uses for a pesticide when the potential exposure level is at least 100-fold lower than the level where there are no health effects, which sets up “a large margin” between legally-allowed amounts and the levels where health effects could occur.
Also, Health Canada noted, “when putting information about atrazine into context,” the product is only used in Canada on commercial fields of corn, sorghum and switchgrass, and at total allowed annual levels that permit it to only be applied once or twice a year.
In the U.S., by comparison, “atrazine is used on more crops and in other areas, at higher application rates, and can be applied more often throughout the growing season than in Canada.” The European Union, on the other hand, has banned atrazine’s use since 2004 after finding levels of the chemical in groundwater monitoring data to be higher than its set threshold.
Canada’s National Farmers Union, in its response to Health Canada’s 2023 proposals, called on the government to further reduce the maximum allowable concentration of atrazine in drinking water and to set a maximum concentration no higher than 1.8 parts per billion of atrazine in groundwater and surface waters.
More recently, in the U.S. in May, atrazine was one of the crop chemicals singled out for criticism in the first report from federal Health Secretary Robert F. Kennedy Jr.’s Make America Healthy Again Commission, which said that in “experimental animal and wildlife studies, exposure to (atrazine) can cause endocrine disruption and birth defects.”
However, that report also cautioned “precipitous changes in agricultural practices could have an adverse impact on American agriculture and the domestic and global food supply” and the government “will continue to regularly review the safety of these important crop protection tools.”
The IARC decision
The IARC emphasizes it’s “a research organization that generates and evaluates evidence related to the causes of cancer but does not make health or policy recommendations,” nor does it “perform risk assessment for any specific exposure scenarios.”
The agency classifies substances “by the strength of the evidence that a substance or agent can cause cancer,” but its classification “does not indicate the level of cancer risk associated with exposure at different levels or in different scenarios.”
- Group 1: Carcinogenic. Shows “sufficient” evidence of causing cancer in people, and of causing cancer in lab animals, and strong “mechanistic” evidence of effects important for cancer to develop in exposed people.
- Group 2A: Probably carcinogenic. Shows “limited” evidence of causing cancer in people, “sufficient” evidence of causing cancer in lab animals, and strong mechanistic evidence of causing cancer in human cells or tissues.
- Group 2B: Possibly carcinogenic. Limited evidence in people and insufficient evidence in lab animals, or inadequate evidence in people but sufficient evidence in lab animals; strong mechanistic evidence.
- Group 3: Not classifiable. Sufficient evidence in lab animals, but strong mechanistic evidence showing the product does not operate to cause cancer in people. Also includes “all other situations not listed above.”
The IARC, back in 1998, had classified atrazine as a Group 3 substance. Last year, however, its advisory group recommended re-evaluating atrazine “with high priority,” based on the availability of new studies.
For atrazine, the IARC’s new monograph cites two case-control studies in which “positive associations” were found between exposure and non-Hodgkin lymphoma cases that were positive for a specific “chromosomal translocation” — but no such associations in non-Hodgkin lymphoma that was negative for that translocation. In other words, the evidence for cancer in humans is “limited.”
In this “chromosomal translocation,” a portion of one chromosome translocates to another, activating a gene that usually inhibits programmed cell death, which in turn can eventually lead to tumours forming.
The IARC also cites “multiple well-conducted studies” showing an increased incidence of malignancies in female Sprague-Dawley and Fischer 344/LATI rats treated with atrazine, as well as “strong mechanistic evidence in experimental systems” that atrazine exhibits certain key characteristics of carcinogens.
The IARC’s Nov. 21 monograph included new classifications for two other crop chemicals. Alachlor, a broad-spectrum herbicide not registered in Canada since 1986, was also classified in Group 2A, while vinclozolin, a fungicide not registered in Canada since 2011, was classified in Group 2B.

Reaction so far
Syngenta, one of the main producers of atrazine, said in a separate statement Nov. 21 that the IARC’s reclassification of the chemical “reflects (the agency’s) mandate to determine if a substance could cause cancer from a potential hazard perspective.”
However, the company said, the IARC “did not establish a causal link between atrazine exposure and an increase in cancer risk.” It added that IARC Group 2A today also includes “very hot beverages, red meat, night shift work (and) occupations such as hairdressing.”
In other words, the company said, the IARC “often places substances with widely differing potencies and very different modes of action — such as smoked ham and the war agent mustard gas — in the same group, which has at times led to confusion in directing public health policies aimed at preventing cancer.”
The IARC’s decision on atrazine specifically, Syngenta said, is “wholly inconsistent with the scientific consensus held by close to 50 regulatory authorities and scientific expert bodies worldwide.”
Among others, the company cited Health Canada’s Pest Management Regulatory Agency (PMRA) and the U.S. Environmental Protection Agency (EPA) as finding “atrazine does not pose any carcinogenic risk and is safe when used in accordance with the registered label instructions.”
BASF and Bayer have not yet issued statements on the IARC ruling.
‘Outsized influence’
The IARC’s reclassification of glyphosate, a somewhat newer but much more widely used herbicide, into Group 2A in 2015 preceded a slew of lawsuits against the chemical’s principal manufacturer: Monsanto, now part of Bayer.
As of this summer, Bayer has so far paid out about US$10 billion and set aside about US$1.39 billion more to settle plaintiffs’ claims that glyphosate, the broad-spectrum active ingredient in the company’s Roundup brand, caused their non-Hodgkin lymphomas and other cancers.
Bayer shareholders have been critical of the company’s acquisition of Monsanto and the resulting exposure to glyphosate-related lawsuits.
In a 2019 paper for the International Association of Defense Counsel, lawyers said the IARC “continues to have an outsized influence on litigation” in such cases.
However, that paper’s authors noted that in at least one U.S. federal multi-district litigation on glyphosate, a court ruling has “recognized that an IARC 2A determination is (by itself) not sufficient for courtroom causation.”
Bayer also continues to refute the IARC’s classification for glyphosate. Bayer’s MSDS for Roundup Transorb HC, for example, acknowledges the active ingredient’s IARC Group 2A classification, but the company adds on the sheet that “our expert opinion is that classification as a carcinogen is not warranted.”
Separately, though, given that the “vast majority” of Roundup-related litigation has come from users in the residential lawn and garden segment, Bayer notes that in 2023 it began to shift its glyphosate products for the U.S. residential L+G market to “new formulations” with “different active ingredients.”
Bayer said it had “taken this action exclusively to manage litigation risk and not because of any safety concerns” and the move would not affect any glyphosate-based agriculture products, nor any Roundup products sold outside the U.S.
















