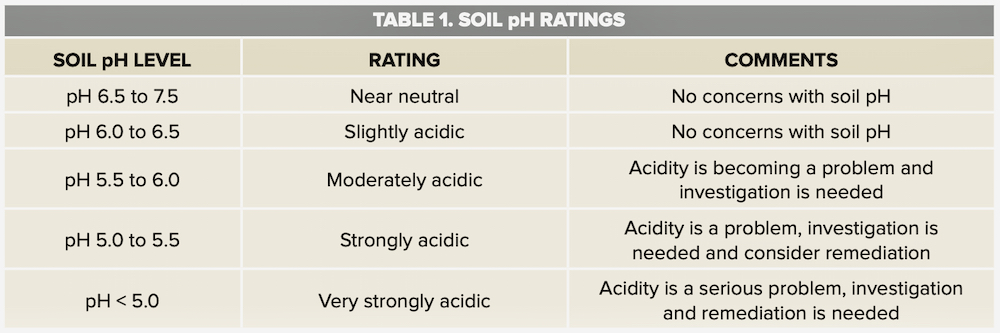
Soil acidity is determined by measuring soil pH. The “H” refers to hydrogen and the “p” refers to hydrogen concentration on a negative logarithm scale (soil pH is -log [H+]).
A soil pH of 7.0 is neutral, meaning the hydrogen (H+) ion concentration in soil equals the hydroxyl (OH-) ion concentration. A soil pH less than 7.0 is acidic and a soil pH greater than 7.0 is alkaline or basic. As soil pH decreases below 7.0, the concentration of hydrogen ions increases and the soil becomes more acidic. As soil pH increases above 7.0, the concentration of hydroxyl ions in soil increases and the soil becomes more alkaline.
Read Also

Southern Alberta farms explore ultra-early seeding
Southern Alberta farmers putting research into practice, pushing ahead traditional seeding times by months for spring wheat and durum
Soil pH value is expressed in log form. When pH is one unit lower, it means 10 times more hydrogen ions in soil solution. A soil pH of 6.0 is 10 times more acidic than a soil with a pH of 7.0 and a soil pH of 5.0 is 100 times more acidic than a soil with a pH of 7.0.
Grainews columnist Les Henry wrote two excellent articles on the topic called, “Acid Soils — A wake-up call,” and “Geography of acid soils in the Prairie provinces.” These two articles are well worth your time to review.
The majority of naturally occurring acid soils in Western Canada are in the grey and dark grey soil zones in Alberta and, to a lesser extent, in northwest Saskatchewan. These soils developed under boreal forest vegetation and are naturally acidic as a result of the acidic leaf litter and organic matter added to the soil over several thousand years of soil development. Some of the solonetzic soil areas of Alberta and western Saskatchewan also have naturally occurring acid soils.
Soil sampling for pH
To accurately assess soil pH in no-till cropped fields, it is best to sample the zero- to 7.5-, 7.5- to 15- and 15- to 30-centimetre depths (zero- to three-, three- to six- and six- to 12-inch depths) separately. This is important to determine the change in soil pH with soil depth. In conventionally tilled fields, sampling the zero- to 15- and 15- to 30-centimetre depths (zero- to six- and six- to 12-inch depths) separately may be adequate.
In uniform fields, 20 to 25 locations should be sampled to assess soil pH. Fields with varying topography and slopes, soil texture or surface soil colour should be separated into unique soil management zones and each zone sampled separately.
Declining soil pH
In recent years, surface soil pH has been gradually declining, particularly in the western Prairies. Surface soil pH used to be monitored by the provincial soil testing labs (Alberta Agriculture lab in Edmonton, the University of Saskatchewan lab in Saskatoon and the Manitoba Agriculture lab in Winnipeg), which tested farmers’ soil samples and tracked soil changes. However, this hasn’t been done for years due to lab privatization. As a result, soil pH changes are not tracked in Western Canada.
There are several reasons for declining soil pH. The long-term use of acidifying nitrogen and sulphur fertilizers is a major factor. For example, when ammonium-based (NH4) fertilizers are applied, nitrogen is converted to nitrate (NO3-) and hydrogen ions are released, causing soil acidification.
Using no-till cropping tends to concentrate acidity in the top 7.5 to 12 centimetres (three to five inches) of soil when nitrogen is side or mid-row banded at depths of five to 10 centimetres (two to four inches). When soil tillage is minimized and nitrogen fertilizer is placed just below the soil surface, soil acidity tends to be more concentrated in the surface soil. In lower-relief areas of fields, increased leaching may occur, resulting in increased acidification in these field areas.
Soil pH affects the physical, chemical and biological properties of soils. Biological activity in acid soils is often reduced, making crop growing conditions less favourable. Wheat yields will start to decline at about a pH of 6 and at a pH of 5.5, wheat yields can be reduced by up to 25 per cent.
The causes of soil acidity damage to crops are complex. As soils become more acidic, forms of aluminum (Al) and manganese (Mn) become more soluble, gradually increasing to toxic levels affecting crop growth. Aluminum toxicity will restrict and distort root growth, reducing nutrient and water uptake. Aluminum will also tie up plant-available soil phosphorus, reducing availability to plants. As soil pH declines from 6.0 to less than 5.0, availability of nitrogen, potassium, sulphur, calcium and magnesium to plants all decline, affecting plant take-up of these nutrients and crop yield.
Survival of rhizobium bacteria can also be seriously affected as soil pH declines to less than 5.5. This affects the ability of legume crops to form nodules to fix nitrogen. Productivity of permanent crops (e.g. alfalfa) and pulse crops (e.g. pea) are greatly reduced as soil pH declines below 5.5.
Some fungal diseases of crops are more prevalent in acidic soils. A good example is clubroot in canola in central Alberta.
Prevention and management
As mentioned, nitrogen and sulphur fertilizers are a major contributor to declining soil pH. Use soil testing to optimize nitrogen and sulphur fertilizer rates to prevent overapplication. Only apply sulphur if really needed. Only apply a split nitrogen application when environmental conditions are favourable for increased yields.
Consider increasing the acreage of pulse crops on your farm. Most pulse crops fix sufficient nitrogen that nitrogen fertilizer is not required. This reduces nitrogen fertilizer use and fertilizer costs. The year after growing a pulse crop, there is often a nitrogen benefit for the subsequent crop, reducing the need for nitrogen fertilizer.
Consider growing alfalfa in your crop rotation for the nitrogen-fixation benefits and to reduce the use of nitrogen fertilizer. Alfalfa has the additional advantage of helping to increase soil organic matter levels and improving soil structure.
If soil pH in the zero- to 7.5-centimetre depth (zero to three inches) is low but the next depth of 7.5 to 15 centimetres (three to six inches) is near neutral, consider trying a tillage operation once every few years to mix the two layers to increase the surface soil pH.
Application of composted manure can be beneficial to maintain or increase soil pH. In our 24-year dryland cropping trial at Bow Island, Alta., we observed that application of composted feedlot manure once every four years increased soil pH by an average of 0.8 over 24 years due to the addition of calcium carbonate in feed rations. However, in treatments that only had nitrogen fertilizer, pH declined by 0.5 over the 24-year period.
In the future, fertilizer manufacturers and dealers may need to consider manufacturing and distributing nitrogen fertilizer products such as calcium ammonium nitrate that are less acidifying versus other nitrogen fertilizer products like urea.
Remediation of acid soils
The best way to remediate acid soil is by application of agricultural lime. The most common product used is calcium carbonate (CaCO3). Other calcium-based products such as calcium hydroxide [Ca(OH)2] and calcium oxide (CaO) can also be used as liming materials.
The application of lime to acid soil will improve the biological, chemical and physical properties of soils. In the 1980s, Alberta Agriculture had a lime subsidy program to assist farmers in central and northern Alberta to purchase and transport lime. This was an excellent program for improving acid soils.
The starting point to remediation is soil testing to determine pH. This is critical to establish the areas or soil management zones in a field where soil pH is low. Then, in the areas that are low, a lime requirement test for each area can be done to determine the ideal rate of lime for each zone.
Some soil testing labs will do a buffer pH test, which is then used by the lab to estimate the rate of lime needed. However, I am not aware of any research in Western Canada that evaluates lime rates based on buffer pH testing. Therefore, I suggest having the soil lab do a “lime requirement test.” From this test, the lab provides the recommended rates required as “pure lime.” It is important to note that agricultural lime sources are not pure. Sources may only be 70 or 80 per cent calcium carbonate, which must be taken into consideration when determining the rate of product to apply. This is called the calcium carbonate equivalent (CCE).
To be most effective, lime must also be very finely ground. The finer the liming material, the greater its surface area, resulting in faster reactivity with the soil. Fineness of the liming material must also be considered in calculating the actual application rate of the liming product.
Ideally, apply lime immediately after harvest to allow time for the lime to react for greatest benefit on soil pH before the next growing season. Lime should be spread very evenly over the soil surface and thoroughly incorporated into the soil. Water is required for the reaction process between the lime and soil. Lime will react more rapidly in a very moist soil versus a drier soil. It often takes several years or more before a response can be measured even under very good soil moisture conditions.
The reaction time will depend on the type of lime used, the fineness or coarseness of the lime material and moisture conditions. Remember that liming materials differ widely in their neutralizing power due to variations in the percentage of calcium and magnesium content. Liming materials with a higher CCE will neutralize soil acidity faster than those with a lower CCE.
How does lime work?
When calcium carbonate is added to an acid soil, it produces carbon dioxide gas and leaves calcium in the soil. The calcium exchanges with exchangeable acidity (hydrogen ions) on the soil exchange complex. The reaction continues with calcium carbonate until all the acidity is neutralized or all the calcium carbonate is used up. The reaction process occurs over several years.
Other calcium-based products such as calcium chloride or calcium sulphate (gypsum) are neutral salts and cannot be used as liming materials and are ineffective in remediating acid soils.
Long-term benefits of liming
The major benefit of liming is increased crop production. Increased crop growth results in more root and plant fibre returned to the soil, which, in turn, will benefit soil organic matter levels in the long term. The addition of lime will reduce soluble aluminum and manganese to non-toxic levels.
Reduced soil acidity will increase the availability of plant nutrients, particularly phosphorus. In strongly acidic soils, phosphorus is retained in less available forms than on slightly acid and neutral soils. A major benefit of liming acid soils is the increased utilization of residual phosphorus by crops.
The increase in soil pH resulting from the application of lime provides a more favourable environment for soil microbiological activity, which improves nutrient cycling. Production of legume crops, such as alfalfa, and pulse crops, such as pea, can be greatly improved due to more favourable soil conditions for the nitrogen-fixing rhizobium bacteria. Forage quality can also be significantly improved.
Starting point
If you think you have reduced crop yields due to acid soils, the place to start is to have problem fields soil sampled to determine soil pH. If a problem is identified, you may want to undertake more intensive field soil sampling and then have lime requirement soil tests completed.
Next, undertake the process to find lime sources and calculate the cost of lime (transportation and application costs) to help determine if application is economically feasible. Finding a good source for lime at a reasonable cost is often a limiting factor.
If the economics look questionable, consider lime application in some test strips on the lowest pH soil areas to assess potential benefits.
For more information on liming, refer to the Alberta Agriculture publication Agdex No. 534-1 Liming Acid Soils available at the Alberta government website.















